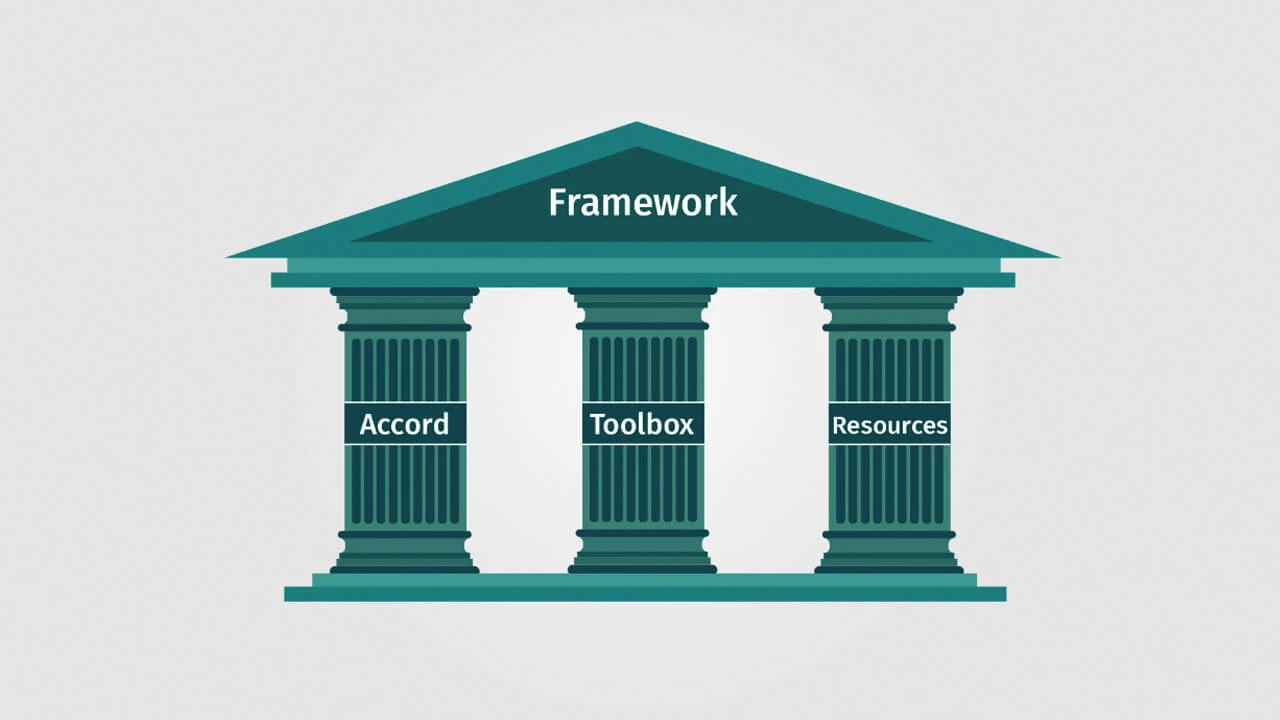
Accord
A statement – The Accord – which lays out the principles for ethical research which we hope all stakeholders can sign up to and endorse. The statement is for all who are concerned to ensure policies are based upon ethical evidence.
Toolbox
A Toolbox to supplement and operationalise the Accord for policy makers and advisors to help them identify ethical evidence that supports their decision-making. It will also help researchers, funders and other stakeholders to produce ethical evidence.
Resources
Additional supportive Resources that complement the Accord and the Toolbox.
The Framework will support policies constructed on ethical evidence, cover the wide spectrum of non-medical research and offer practical solutions for all evidence-seeking stakeholders, that will comply with the highest standards of research ethics and integrity. The Framework has been explored and tested with the appropriate constituencies and across the full range of stakeholders. These include the producers of research, disseminators and intermediaries, influencers, policy advisers, decision-makers and implementers.
To answer the “Who is this for?” question: it is for policymakers keen to use ethical evidence; it is for policy advisors seeking to offer advice based on responsible sources; it is for researchers and their funders wishing to make sure that policies will be based upon their ethically produced evidence; it is for think tanks wishing to enhance their legitimacy by demonstrating that their reports have been produced with integrity and it is also available for citizens to make their own assessment about the evidential sources of the policies that directly affect them.
The STEP (Scientific, Trustworthy, and Ethical evidence for Policy) ACCORD
As signatories to the Accord
- We recognize that an underpinning by high quality research, analysis and evidence, including policy appraisals and evaluations, is a pre-condition for evidence-based policy-/decision-making, and hence rational policy actions and effective outcomes.
- As individuals and institutions involved in commissioning, funding, sponsoring or conducting research, collecting or using evidence for policymaking, we aim to be as transparent as possible on how the high quality of that evidence is assured and will flag up any potential conflicts of interest.
- We agree that to a reasonable degree the independence and integrity of individuals responsible for the conducting and/or gathering of research evidence and its use in policymaking must be respected and supported in ways that ensure the evidence they produce is neither biased nor misleading.
- We will communicate, employ and/or apply only high quality evidence, research or enquiry, in other words evidence that has been undertaken, gathered, collated and analyzed using sound, robust and ethical methods appropriate to the task.
- We will ensure that the commissioning, funding, management, conduct, dissemination and governance of research meet high standards of ethics and integrity.

Putting The Accord into practice: Some Recommendations
To help implement the Accord and the principles behind it users need to know:
- How to conduct research ethically and with integrity (for researchers, managers and funders).
- How to ensure research is conducted ethically and with integrity (for reviewers in research ethics appraisal).
- How to supply evidence for effective policymaking (for researchers, managers, funders).
- How to select good quality research (for science/policy advisors and policymakers).
- How to evaluate the ethical impact of policies (for ALL above stakeholders).
The PRO-RES Toolbox is designed to help implement these recommendations.
The PRO-RES Framework
Decision takers and policymakers should be seeking evidence to support their work from the range of expertise on offer. Sound, reliable, transparent research, not driven by ideology or subservient to it and undeclared vested interests, produces robust evidence that can benefit social wellbeing and societal progress. It is in the interests of the scientific community to ensure the evidence produced is reliable and trustworthy and ethically generated. It is in the interests of those who make policy to be able to assure the decision takers (and the general public) that evidence has been generated in the best possible way.
We present here a draft statement of principles that lie behind seeking/using ethical evidence from non-medical research to inform policy. The short, clear, succinct and actionable statement we present here is designated the ‘Accord’. This is the baseline that we intend the further consultation process to be built on.
Neither its title nor content is ‘fixed’ at this point. The Accord will be accompanied with further tools and information/resources, thus constituting the PRO-RES Framework for non-medical research.
We aim to explore its potential with the appropriate constituencies and across the range of stakeholders. These include the producers of research, disseminators and intermediaries, influencers, policy advisers, decision-makers and implementers. The draft Accord is based on the work accomplished by the first phase of the PRO-RES Project and based on declared foundational assumptions about the values, principles and standards involved in ethical research conducted with integrity.
What do we mean by Continuous Discursive engagement
- There needs to be an ethical discourse to be sure that researchers are aware of, and sensitive to, the ethical dimensions of their work. That awareness depends on engagement in ethical discourse as an integral aspect of engagement in research.
- To bring about a cultural change in research activity, theremustbe engagement of everyone responsible for the process, including researchers, stakeholders, peers and the users of research.
- This engagement needs to be continuous. Ethical issues can arise at every stage of research: conception, development, proposal, process, conclusion, dissemination and use. Ethical consideration cannot be a single-stage process.